Fluorescence Spectrometer Develops Organic Room-Temperature Phosphorescent Materials
This article mainly describes the application of fluorescence spectrometer in the development of organic room temperature phosphorescent materials.
Research Background
On Earth, the photosynthesis of green plants is a continuous process, and a key step in photosynthesis is the charge transfer (CT) process between the cell membrane and protein assemblies. Relying on long-lived CT states, multi-step reactions in living organisms can proceed smoothly. In fact, long-lived CT states are crucial for the effective occurrence of processes such as photosynthesis, photocatalysis, and organic photoelectric conversion. Therefore, developing single-molecule ultra-long organic room-temperature phosphorescent materials (SMUOP) that possess CT transition properties (3CT), high energy levels (>2.0 eV), and long lifetimes (>0.1 s) is of significant research value. Unfortunately, existing materials of this type mostly exhibit radiative transition properties of localized excited triplet states (3LE), making it necessary to explore molecular construction strategies for 3CT-type SMUOP materials with high energy and long lifetimes.Research Results
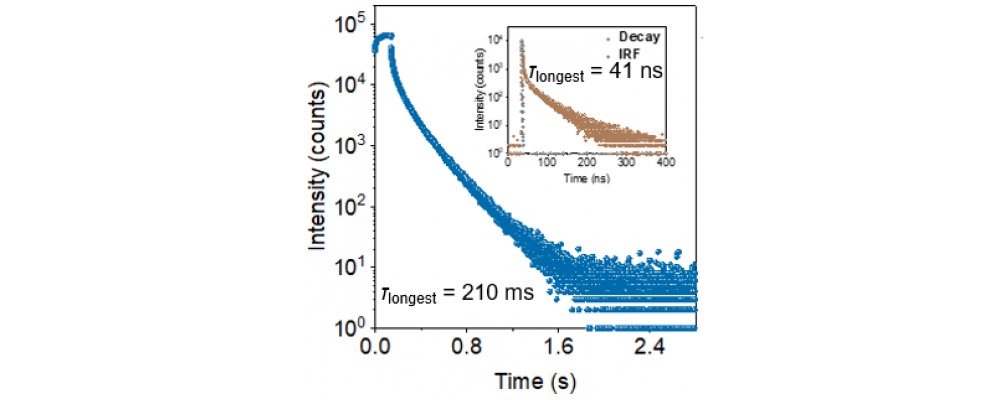
A molecular structure design strategy for 3CT-type SMUOP materials has been proposed, which involves connecting electron donor (D) and acceptor (A) units through sp³-hybridized carbon atoms (sp³-C). This design creates a D-CH₂(sp³)-A type molecule where the D and A units can only undergo spatial electronic coupling, preventing effective through-bond electronic coupling via conjugated bridges. As a result, the phosphorescent radiative transition process from the formed 3CT state back to the singlet ground state (S₀) exhibits stronger forbidden characteristics, facilitating the acquisition of long-lived 3CT states. The compound NIC-DMAC, constructed based on this strategy, achieves a 3CT state with a lifetime of up to 0.21 s, even in a less rigid poly(methyl methacrylate) (PMMA) matrix at a doping concentration of 1.5 wt%, while its 3CT energy level can reach as high as 2.5 eV. 
Instrument Contribution
The research utilized the HORIBA Fluorolog-3 research-grade fluorescence spectrometer to test the fluorescence lifetime (τF, shown by the yellow curve in the figure) of the same thin film sample of the target compound under air conditions, as well as the phosphorescence lifetime (τPh, shown by the blue curve in the figure) under vacuum conditions. By using these two lifetime parameters, along with the sample's photoluminescence quantum efficiency data, the fluorescence radiative transition rate constant (kF) and phosphorescence radiative transition rate constant (kPh) were calculated for the compound.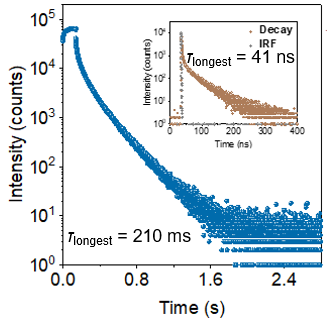 Fluorescence lifetime (τF) of the same thin film sample of the target compound under air conditions and phosphorescence lifetime under vacuum conditions.kF is a core parameter of this work, reflecting the allowed nature of the radiative transition process of the compound's 1CT state. The calculation results indicate that kF for NIC-DMAC is only 1.9 × 10⁶ s⁻¹, whereas for common TBCT molecules, this parameter typically reaches the 10⁷ range. This supports our hypothesis that the radiative transition process of TSCT-type excitons has a greater forbidden character compared to TBCT-type excitons.
Fluorescence lifetime (τF) of the same thin film sample of the target compound under air conditions and phosphorescence lifetime under vacuum conditions.kF is a core parameter of this work, reflecting the allowed nature of the radiative transition process of the compound's 1CT state. The calculation results indicate that kF for NIC-DMAC is only 1.9 × 10⁶ s⁻¹, whereas for common TBCT molecules, this parameter typically reaches the 10⁷ range. This supports our hypothesis that the radiative transition process of TSCT-type excitons has a greater forbidden character compared to TBCT-type excitons. 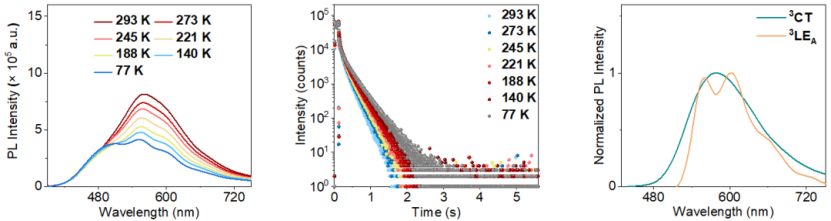



Steady-state photoluminescence spectra, time-resolved emission spectra, and lifetimes of the target compound dispersed in PMMA under vacuum conditions at different temperatures.
The HORIBA Fluorolog-3 was also used to test the steady-state photoluminescence spectra, time-resolved emission spectra, and lifetimes of the target compound dispersed in PMMA under vacuum conditions at different temperatures. As shown in the left panel of the figure, the phosphorescence emission band of this sample exhibits "thermal activation" properties, and as the temperature increases, the fine structure of the phosphorescence emission band becomes progressively blurred. This indicates that the 3CT-type room-temperature phosphorescence of this sample originates from its higher energy excited triplet states (Tn), rather than from the radiative deactivation of T1 excitons. The middle panel shows that the phosphorescence lifetime of the sample slightly decreases with increasing temperature. The right panel indicates that the T1 excitons of this sample exhibit 3LE transition properties. Combining these experimental results, we conclude that at room temperature, the 3LE-type T1 excitons can convert to 3CT-type Tn excitons via a reverse intersystem crossing process.
The Fluorolog-3 research-grade fluorescence spectrometer used in this study has now been upgraded to the Fluorolog-QM™ modular time-resolved fluorescence spectrometer.
At the same time, the research utilized the HORIBA FluoroMax+ high-sensitivity integrated fluorescence spectrometer to test the room-temperature phosphorescence spectra of the target compound dispersed in matrices of different polarities (as shown in the figure: PS represents polystyrene, and PMMA represents poly(methyl methacrylate)). It was found that the phosphorescence emission properties exhibit a positive solvent effect, indicating that the room-temperature phosphorescence of NIC-DMAC indeed possesses 3CT transition characteristics.
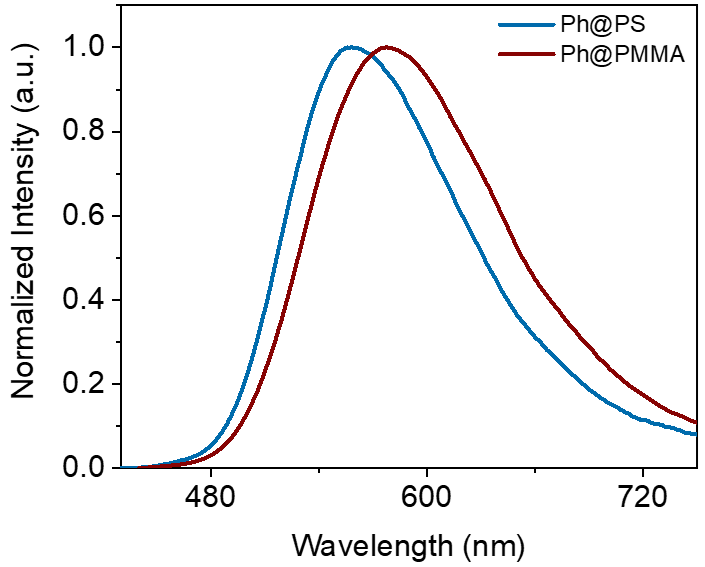
Room-temperature phosphorescence spectra of the target compound dispersed in matrices of different polarities.
Additionally, under the same testing conditions, the HORIBA FluoroMax+ measured the steady-state photoluminescence spectra of the same thin film sample of the target compound in air and vacuum to determine the ratio of luminescence intensity in vacuum to that in air. Based on this ratio and the photoluminescence quantum efficiency (ΦAir) of the target compound's film sample measured in air using the FL-3, which is 7.6%, the luminescence quantum efficiency under vacuum (ΦVac) was calculated to be 13.7%. By calculating the difference between ΦVac and ΦAir, the phosphorescence quantum efficiency (ΦPh) of the material was determined to be 6.1%. This data indicates that our method not only achieves long-lived 3CT states but also enables these states to exhibit a relatively good luminescence quantum efficiency.
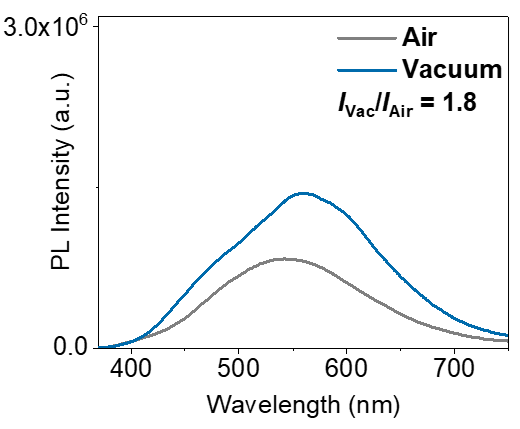
Steady-state photoluminescence spectra of the same thin film sample of the target compound in air and vacuum conditions.
"Our research requires testing the steady-state emission spectra, time-resolved emission spectra, photoluminescence quantum efficiency, and emission lifetimes of the samples. The modular instrument design allows for multiple tests to be conducted on the same device, significantly improving work efficiency," researchers stated.
Another instrument used in the research is the HORIBA FluoroMax+ high-sensitivity integrated fluorescence spectrometer.
Future Science
"The future holds infinite possibilities. From a smaller perspective, we hope our findings can provide guidance for artificial photosynthesis and the photovoltaic field, and serve as a reference for addressing the future energy crisis," researchers stated.





















Comments: 0
No comments