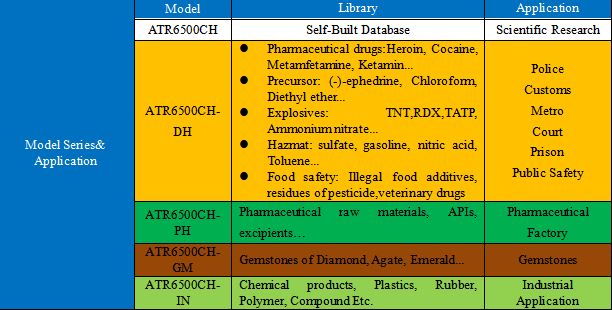
1st, ATR6500CH upgrade on ATR6500 winnder-award instrument in Y2020 with reliable CE certification and Police certificate.
2nd, Fast ID of Narcotics, Explosives, Drugs, Liquid, Food, Gemstones, and Plastics in states of powder, liquid and solid.
3rd, Super-Speed and takes few seconds can match accurate results exporting reports in pdf,csv,txt format.
4th, Advanced algorithm can detect mixtures.
5th, Pretreatment free and nondestructive to the samples to be detected.
6th, OEM customization.
7th, Free Software PHARM-ID & Police-ID and upgrade free.
8th, Barcode Scan
9th, Mini Printer optional spare part
10th, Free basic library database, total Library >10,000 entries optional
11th, Rechargeable battery for field detection allow continuous operation
12th, The toxic and hazardous gases alarm function
13th, Readout altitude sensor
| Raman Spectrometer System Parameters | |
| Interface | WIFI, USB, Type-C, Bluetooth,GSM. |
| Operating System | Android |
| Operating temperature | -20 – 50 ℃ |
| Dimension | 172×85×30 mm |
| Weight | 480g |
| Touch Screen | 5.5",1920×1080,Multi-point touch control |
| Export Report | Test results report export pdf,csv, txt including detect result, spectral info,evident,pictures. |
| Battery | 8 hours, replaceable battery for unlimited battery life |
| Charging Type | USB Type-C |
| Library | self-built library |
| Raman Spectrometer Optical Parameters | |
| Spectral Range | 200-4000 cm-1 |
| Resolution | 10 cm-1 |
| Raman spectrometer Detector | |
| Detector Model | Hamamatsu CCD |
| Raman Spectrometer Laser Parameters | |
| Excitation Wavelength | 785 ± 0.5nm |
- Replaceable Battery for unlimited life span;
- Various toxic and hazardous gases Alarm;
- Built-in altitude sensor read out;
- Non-Destructive, Fast Detect & Identify, One-touch Operation;
- Advanced algorithm, mixture detection;
- HD 5.5" capacitive touch screen, smooth operation system;
- double cameras of 13-mega plus 8-mega;
- Barcode & QR scan
- Precision GPS positioning;
- Multiple modes of 4G, GPS, GPRS, Bluetooth, WIFI
- Self-Built Spectral Library
- Test results report export pdf
- Built-in Li battery battery
- Super-Thin & Lightweight(450g)
- Easy-to-Carry to field test
- IP-66 compliant
- Public Safety;
- Food Safety;
- Dangerous Chemicals Detect at High-Speed Train, Metro Entrance
- APIs & Pharmaceutical Excipients;
- Gemstones & Materials Identification,
- Mineral sorting
- Experimental Research;
- Antique authentication;
Q - What is PalmRam?
A - Optosky's newly launched ATR6500CH is upgraded on the basis of best-seller ATR6500, and not only reserves the advantages of super-thin, ultra-light, fast ID, but also makes it a rechargeable battery for field detection extending battery life. The toxic and hazardous gases alarm and readout altitude sensor.
Q - How to use PalmRam in the pharmaceutical Industry?
A - In pharmaceutical production, Raman spectrometers are suitable for the inspection of incoming raw materials, the allocation of materials during API production, and the identification of counterfeit and inferior products.
Pharmaceutical manufacturers use Raman spectrometers for raw material analysis in pharmaceutical manufacturing processes. Even non-professional operators can use hand-held Raman analyzers to quickly and accurately identify raw materials.
QA / QC applications for Raman analyzers include enhanced raw material authenticity checks for similar compounds, multi-component authenticity checks, and identification and quantification of intermediate and finished products. In PAT, specific applications include on-line end determinations for distillation, reaction monitoring, and powder mixing operations.
Counterfeit or substandard drugs are a growing problem worldwide. To protect patient and brand integrity, pharmaceutical manufacturers use portable Raman analyzers to identify counterfeit products. The portable Raman analyzer allows users without chemical training to conduct on-site screening of drug samples and quickly and accurately identify counterfeit or substandard drugs. Because the spectrum generated by PalmRam is able to check all the ingredients in the pharmaceutical dosage form: API, excipients, fillers, dyes, coatings, etc. to produce a spectrum representing all ingredients (and their relative concentrations), any slight deviations from the original Both will cause the obtained spectrum to change. This makes ATR6500CH the ideal tool to quickly detect counterfeit and inferior medicines in the field.
ATR6500CH integrates built-in battery replaceable with unlimited battery life facilitate the operators long time operation in the pharmaceutical factory.
Q - What is the correlation of Raman spectroscopy with high-throughput screening in the pharmaceutical industry (HTS) ?
A - High-throughput screening (HTS) used in the pharmaceutical industry involves testing the collection of hundreds of samples and subsequent analysis to quickly determine the biological or biochemical activity of a large number of drug-like compounds. HTS technology is used to characterize polymers and drugs by integrating itself into the manufacturing process to provide real-time measurements. Due to the large number of samples, HTS requires a high degree of automation and a small number of sample preparation steps. Raman spectrometers meet this need and are widely used to automate HTS and analytical measurements.
Q - Can only scientists or experts use Raman technology in pharmaceutical and pharmaceutical production?
A - Some handheld Raman analyzers used in the pharmaceutical industry are specifically designed and set up for non-expert users. For example, the ATR6600 pharmaceutical handheld Raman analyzer can not only obtain the Raman spectrum of the target material, but also determine the measurement uncertainty in real time, giving relevant factors such as sample characteristics, instrument telemetry, environment, and test environment. End users of field material identification systems are usually not spectroscopic experts, so instrument data can be relied upon to convert instrument data to qualitative results.
Q - Is Raman spectrosopy suitable in USP & EP?
A - The ATR6600 Handheld Raman Spectrometer fully complies with the revised standards USP General Rules for Raman Spectroscopy <1120>. The ATR6600 enhances 21 CFR Part 11 compliance security features, such as biometric login and optional password aging and complexity, allowing users to customize the analyzer's security settings beyond regulatory requirements.
Q - Can Raman spectrometers be used to analyze tablet film coatings?
A - Pharmaceutical companies that produce tablets, capsules, and other solid dosage forms need to use film coatings on their products to differentiate the visual appearance, improve swallowing ability, and mask unpleasant tastes or odors. Film coating can also reduce cracking and chipping of tablets and provide protection from light, humidity and ambient gases. The latest handheld Raman analyzers have built-in multivariate residual analysis decision tools to identify most materials, while more complex materials analysis requires users to build custom advanced algorithms. Some handheld analyzers use built-in chemometric configurations to enable users to develop customized predictive applications, including classification, semi-quantitative, and quantitative methods, to establish pass / fail criteria to identify highly similar compounds such as tablet packs clothes).
Q - Can Raman spectroscopy be used to verify stearates and other pharmaceutical capsule lubricants?
A - Magnesium stearate is a white powder that turns into a solid at room temperature. In pharmaceutical manufacturing, magnesium stearate is the most commonly used capsule and tablet lubricant to help prevent pharmaceutical ingredients from adhering to manufacturing equipment. Calcium stearate, and to a lesser extent, zinc stearate, are also used as pharmaceutical excipients in the production process, mainly for the lubrication of tablets and capsules.
Magnesium stearate, calcium stearate, and zinc stearate have similar compound structures, so validation during the feed inspection process is more challenging. Although some pharmaceutical industry handheld Raman analyzers have built-in multivariate residual analysis decision tools to identify most materials, more complex materials analysis requires users to build custom advanced methods. Those using a built-in chemometric configuration of the Raman analyzer enable users to develop customized predictive applications, including classification, semi-quantitative, and quantitative methods, enabling users to build models that can be applied to the analyzer.
Q - Can Raman spectrometers analyze drugs through packaging?
A - Yes, the latest handheld analyzers can scan through plastic bags, glass containers, clear plastic covers, and clear plastic covers. These analyzers' point-and-measure sampling is non-contact and non-destructive, minimizing the risk of cross-contamination and operator exposure.
Q - Is Raman spectroscopy method dangerious?
A - In Raman spectroscopy, a sample of unknown material is illuminated with a monochromatic (single wavelength or single frequency) laser. The danger of using high-power lasers must be recognized, especially when their wavelengths are in the near-infrared region of the spectrum, because they are not visible to the naked eye at this time. Fiber optic probes should be used with care and refer to relevant government regulations regarding lasers and laser classification.
The laser used in Optosky's ATR6600 is Class IIIb under the FDA CDRH classification system. Never point the instrument at yourself or others. Do not start the instrument unless a sample completely covers the laser hole. Before removing the sample from the laser hole, be sure to stop the measurement first. The manufacturer's user manual provides specific country regulations to which the analyzer laser complies.
Q - Is Raman spectroscopy destructive to pharmaceutical materials?
A - Although Raman is generally considered a non-destructive technique, conditions such as exposure time, laser power, and sample properties can also cause sample degradation. The energy transmitted by the laser depends on the exposure time and wavelength. It may change the physical state and may damage the sample.
Q - How long test time can be delay of ATR6500CH?
A - ATR6500 can delay 30 min, which is safe for detection of TNT,RDX,TATP, Ammonium nitrate...
What are the Reasons for ATR6500CH built-in battery as a future trend:
1. Built-in battery make the instrument thinner and compact size, ATR6500CH shall be the most compact model
2. Big touch-screen & Intelligent instrument design decide the built-in battery trend.
3. The battery capacities are larger than the old version rechargeable battery. ATR6500CH is a new version of a rechargeable built-in battery.
4. The rechargeable battery adds a spare part for customers' selection.